Isolators Aseptic Isolator Aseptic Isolator Basic Features The Aseptic Isolator is designed to the challenges and market requirements for low OEL containment systems in Pharmaceutical,...
Read MoreProject Overview
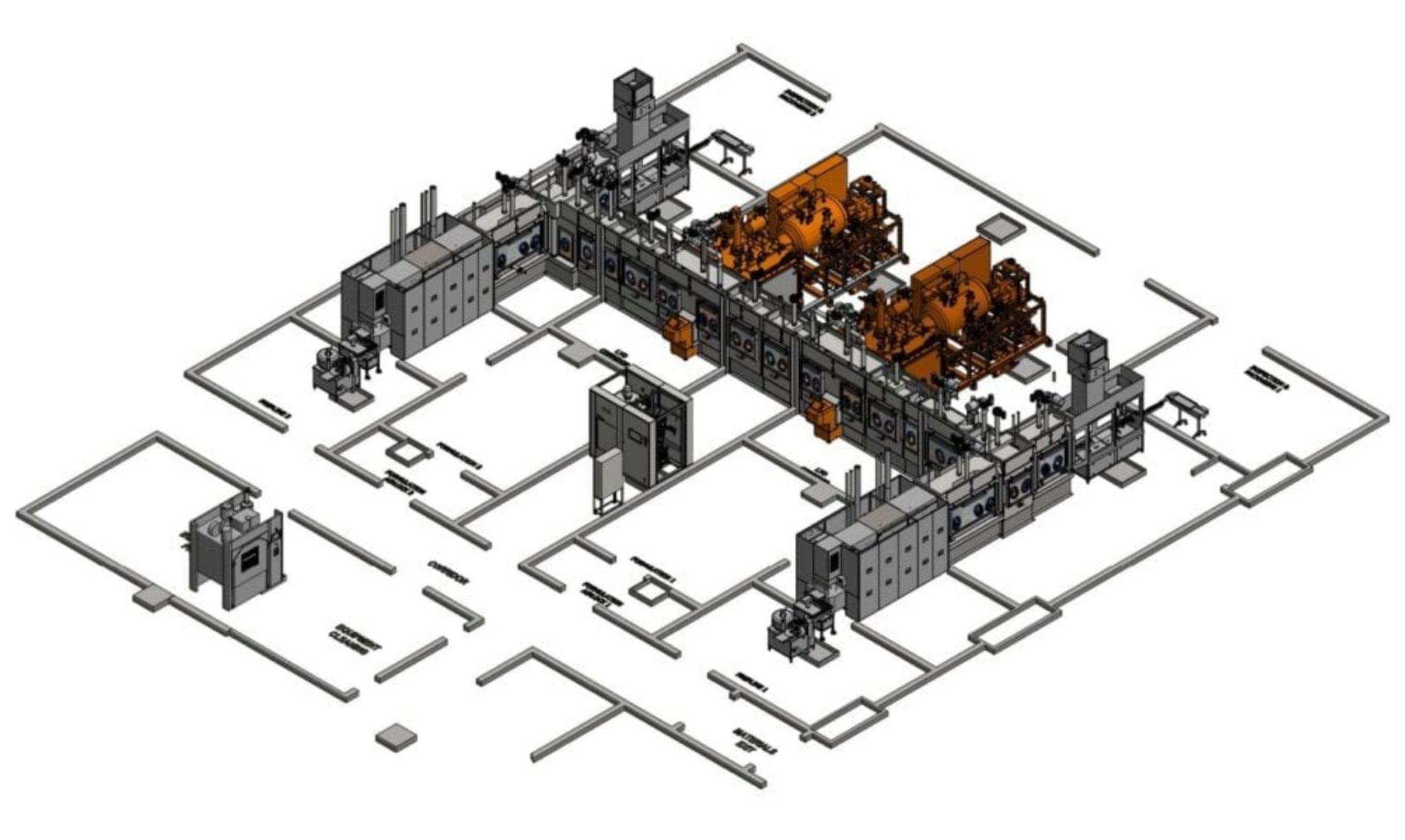
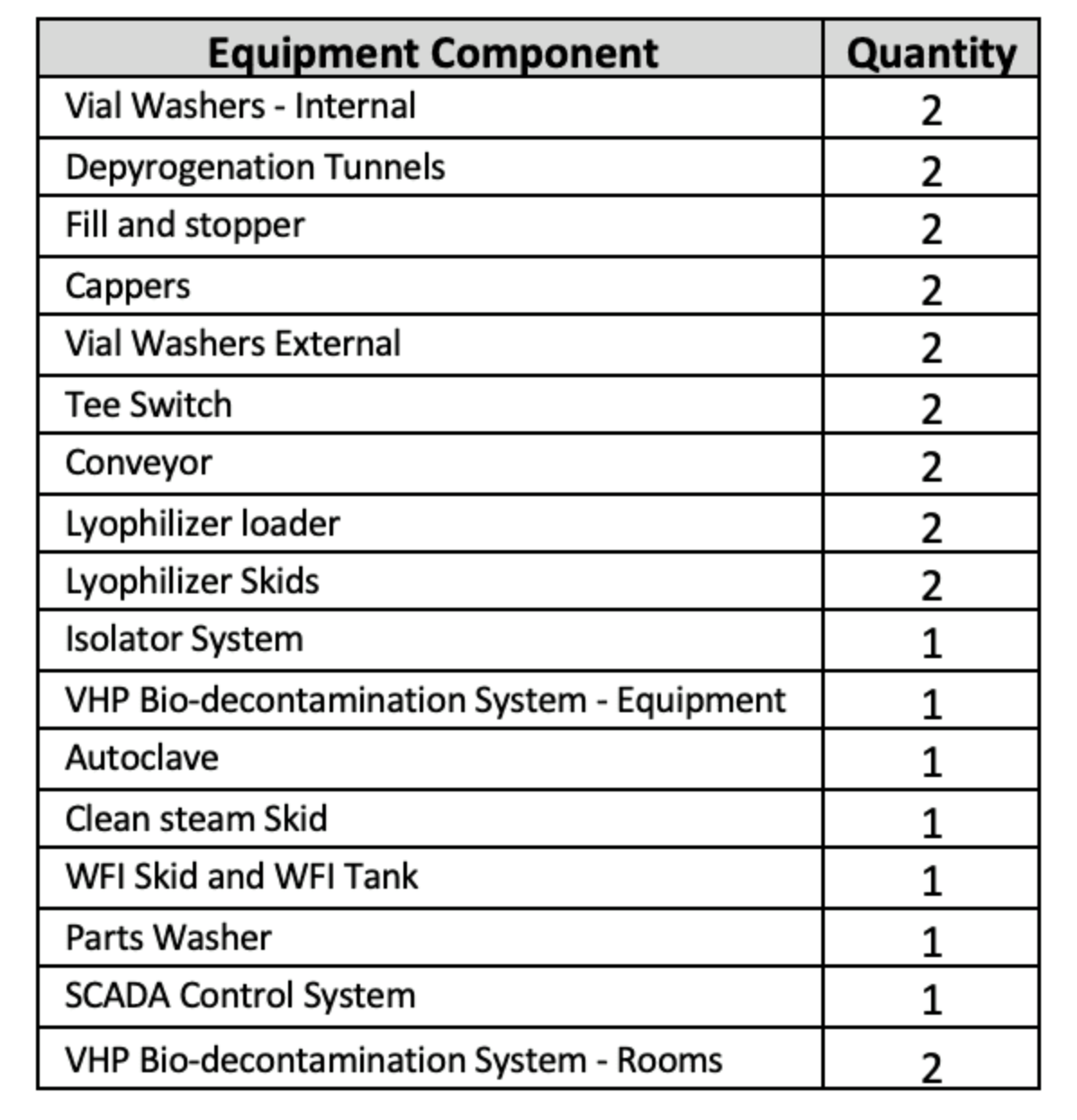
This system is one of the largest automated sterile fill/finish systems in existence to date and the most flexible system in terms of batch scale and vial sizes ever undertaken. The system is two identical mirror-image fill-finish lines capable of operating in 14 different configurable modes of operation. The extreme flexibility designed in the system is intended to accommodate the unique business model of the University that caters to performing clinical trial batches yet will be expandable to take on higher volume production runs in the future.
Less than 30% of the system is manufactured by CPS (Isolators, Controls and integration elements) with the rest purchased from a network of major specialist subcontractors under control of the CPS Project Management and Technical Services team.
The system operates through a SCADA system compliant with FDA 21CFR Part 11 for electronic signatures and records. Both lines are fully automated with operator intervention limited to only to load and unload vials from the system, set-up preparation and cleaning post batch.